For decades, accessing and sharing health data has been a persistent challenge for patients and healthcare professionals and life sciences organizations across the European Union.
Patients face multiple hurdles when managing their data and sharing it with medical professionals, from poorly implemented apps and limited interoperability for electronic patient records, to low digitalization across most European health systems.
Similarly, healthcare organizations face significant barriers to accessing patient data for research and innovation. Too often, patient health data is restricted to local systems and isn’t easily shareable due to uneven GDPR implementations throughout the EU.
But this is all about to change. The European Health Data Space Regulation (EHDS) aims to establish a common framework and infrastructure for the use of health data for research, innovation, policy-making, and regulatory activities in the EU
It’s designed to give patients greater control of their personal health data and make EU health data more accessible to healthcare and life sciences organizations. If embraced in the right way, EHDS has huge potential to advance innovation in European healthcare and solve some long-standing challenges.
But to make the most of the opportunity, you’ll need to have the right data strategy and infrastructure in place to enable seamless data sharing.
What’s possible with the EHDS
The core premise of the EHDS is to unlock the full potential of primary and secondary use of health data; primary use involving patients’ control of data, and secondary covering the use of health data for research, innovation, and policy creation.
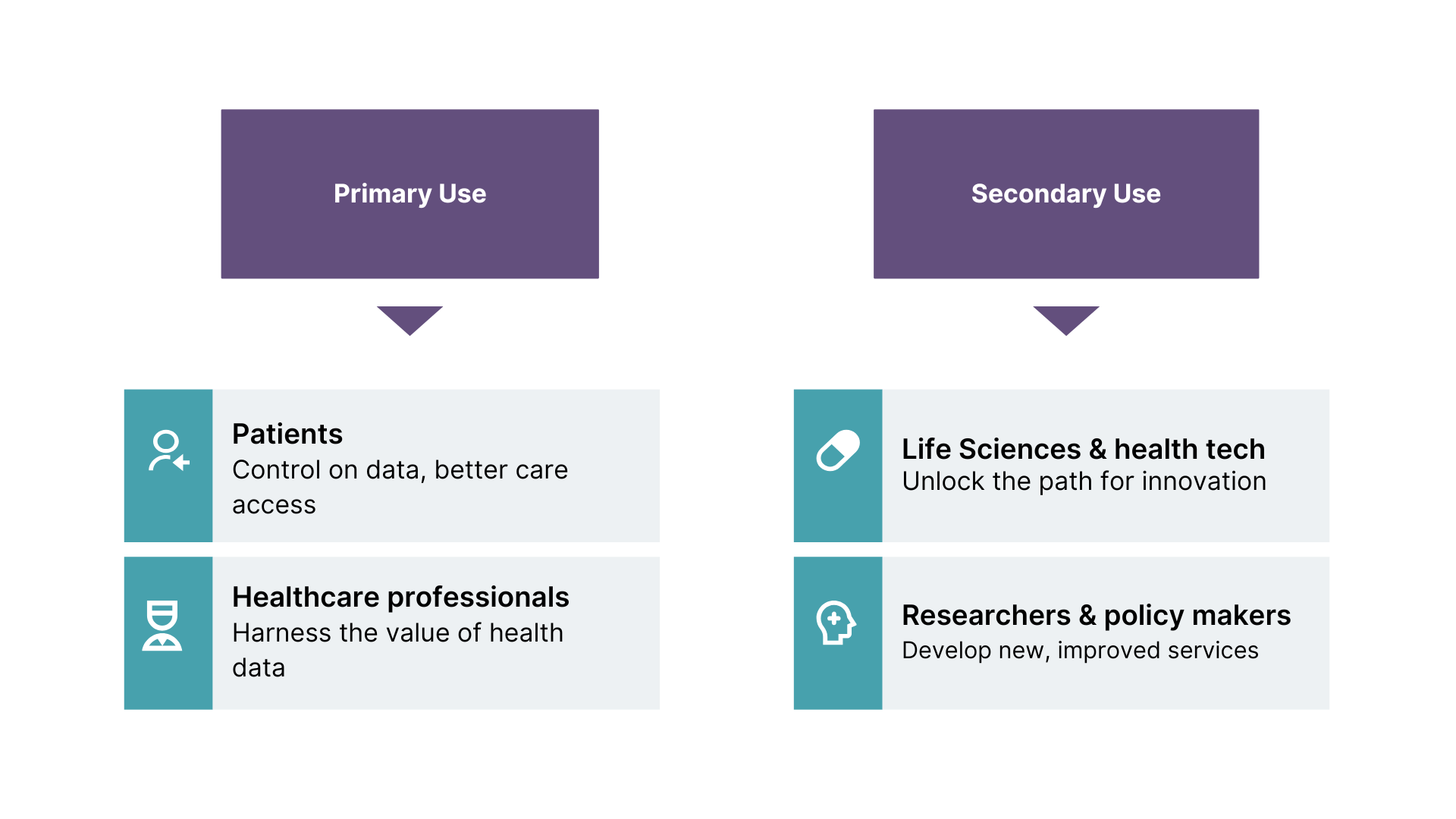

For primary use cases, EU citizens will be able to exercise their rights to data access, gaining the ability to control and share their health data themselves. These capabilities will be particularly important for care that spans multiple countries and multiple regions in decentralized states, such as when a patient needs to visit a specialist site for treatment of rare diseases, or when a cancer patient needs a second opinion on their diagnosis.
In these cases, patients will be able to easily and securely share their data with healthcare professionals in different settings, all within a trusted governance framework based on clear rules and standards. The regulation will also give patients greater control over their data, including the ability to restrict access to it, and access information about how their data is being used.
Arguably, EHDS will have the most significant impact on the secondary use of health data. With simplified and regulated access to data sets within and across borders for commercial use, healthcare and life sciences organizations will significantly expand the volume and type of data they can use in their research and development of new healthcare solutions.
This will include data sets such as:
Electronic health records — to reduce the administrative pressure and restrictions placed on healthcare professionals when procuring patient records.
Genomics data — to improve the diagnosis, treatment, and research of genetic diseases.
Health data from clinical trials — to reduce the need for repeat trials and accelerate validation cycles for new drugs and healthcare solutions.
Real-world data from medical devices and wellness applications — to advance drug development and identify new treatment options for patients.
Ultimately, EHDS has the potential to advance innovation in every corner of European healthcare, unlocking opportunities for every stakeholder, from patients to researchers and frontline healthcare providers, regulators and policymakers.
Building the right data strategy for internal and external collaboration
While the potential of EHDS is massive, many healthcare and life sciences organizations won’t currently be in a position to embrace it.
Data interoperability is a core fundamental of EHDS, and historically, it’s been a major challenge in European healthcare. But rather than seeing this as a barrier to EHDS, organizations should instead view it as an opportunity — an opportunity to solve the problems of data silos, data quality, aging infrastructures, and low interoperability that have previously held them back.
Given the complexity, variance of both systems, platforms and data for healthcare and lifesciences, a need for polyglot data architecture emerges. This may require combining approaches such as data as a product, data products or data mesh.
Data mesh provides a robust solution to these problems, and conveniently, shares a lot of its foundational principles with those of EHDS. These include:
Domain ownership — which allows ecosystems to scale as its number of use cases scale.
Data as a product— allowing users to easily discover, understand, and use high-quality data.
Self-serve data infrastructure as a platform — enabling domain teams to create and consume data productions autonomously.
Federated computational governance — with an ecosystem of shared interoperability standards built into platforms at the code level.
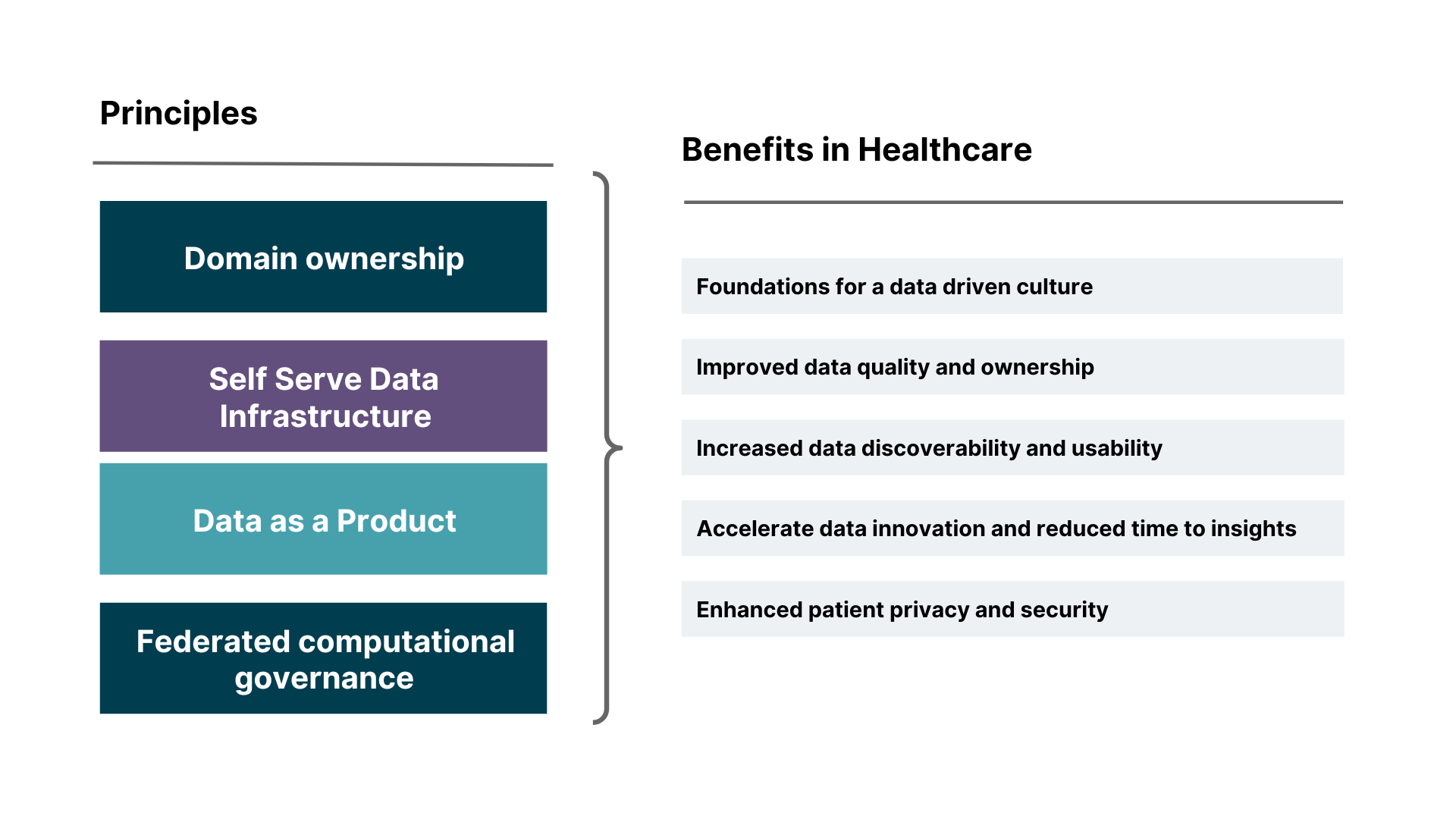

Similar to the EHDS, using a data mesh approach for the healthcare sector aims to create a data-driven culture. They demand that participant organizations — and the domains within them — set and uphold strong standards for data quality and usage. In a data mesh, data sharing is inherently federated. Each domain, representing a specific area of expertise (e.g., a hospital, a research institution), manages and shares its data as independent products. This allows for greater autonomy and flexibility, as domains can control how their data is accessed and used.
It also reinvents the traditional way of thinking about health data. In a data mesh, data sharing is inherently federated. Each domain — representing a specific area of expertise such as a hospital, a research institution, or a specialized team — manages and shares its data as independent products. This allows for greater autonomy and flexibility, as domains can control how their data is accessed and used.
Data from multiple sources is integrated and transformed to create data products for specific use cases. In healthcare, a good example would be building a genomic research data product that unifies processed, analyzed, and curated sets of genomic data from diverse sources.
Federated governance guardrails ensure that the domain closest to each data product retains ownership and control over it. Meanwhile, policy as code enables data products to be securely — and in most cases, anonymously — shared with known third parties, enabling them to utilize collated data for their own projects, without compromising the security or privacy of underlying data sets.
Robust data platforms provide a space for the sharing and exchange of data, and contain the infrastructure, tools, and services necessary for creating, hosting and consuming these health related data products. This includes technologies for data storage, processing, analysis and governance.
The availability of high-quality data and the possibility of using, combining and reusing data from various sources will be particularly useful for complex analytics and training AI and machine learning models. Whether the models are being used to demonstrate the results of pharma research or directly serve patients, their effectiveness is completely dependent on the data they’re trained on, so unlocking access to more data products in the EHDS will only accelerate their capabilities.
Start preparing your organization for the EHDS
Data sharing within the EHDS has huge potential to advance European healthcare, and approaches such as Data as a Product, data products or data mesh may prove to be an instrumental factor in its success.
Together, Thoughtworks and AWS specialize in data strategies and implementation, including data mesh at scale tailored for healthcare settings. We’ve seen first-hand how they can boost collaboration between healthcare stakeholders and organizations. We also understand the challenges and opportunities EHDS will bring — and what healthcare organizations will need to do to embrace them.
If you'd like to discuss your health data management challenges — and how EHDS and data mesh could help you solve them — get in touch with us today.



















